Repurposing non-research facilities to house specialized life sciences and therapeutics labs is a growing trend sparked by real estate inventory and the fiscal practicality of renovation rather than new construction. The vacancy rate throughout the United States for lab space in the fourth quarter of 2022 was 6 percent versus 19.5 percent for office space, according to the Jones Lang LaSalle real estate transparency index. The vacancy rates for 2021 were less than 4 percent for labs and approximately 15 percent for offices.
“There are not a lot of spaces suitable for life sciences in many regions,” says Prashant Gongal, senior project architect at Perkins&Will. “The life sciences and therapeutics industry started booming pre-pandemic, and during the pandemic there were a lot of vacant office buildings. Additionally, it is sustainable and economical to repurpose or reposition a building to life science rather than build a new one.”
The vacancy rates and the evolution of cell therapy, gene therapy, and viral vector processes to treat diseases have increased the need for specialized research spaces, such as cGMP lab cleanrooms. Gongal is seeing more developers purchasing office space and converting it to research labs, even in situations where a renovation might not seem feasible.
He says organizations can successfully build a cGMP space in a constrained area, such as a 12-foot floor-to-floor space that does not have a traditional interstitial ceiling to house distributional infrastructure. Developers can realize real estate cost savings and an overall life cycle cost reduction by taking the following steps:
- Using new technologies and proper planning for close proximity to central MEP systems
- Planning and using building information modeling (BIM) to access and maintain mechanical systems, other services, and resources
- Sharing mechanical space for science and technology programs
“Ideally, we want to have a space that is 16 feet floor to floor so we can fit all the MEP features,” says Gongal. “It’s also good to have interstitial space; good load-bearing floor slabs to withstand the equipment load; increased MEP and HVAC capacity; and intricate plumbing, monitoring, and security systems, all requiring a lot of ductwork, piping, conduit, and cables.”
However, ingenuity, creativity, and new technologies can be used to design a cGMP facility as part of a renovation in older buildings that do not have the desired features.
Repurposing NIH Building to House a cGMP Lab
Renovation of the E-Wing of Building 10 on the Bethesda, Md., campus of the National Institutes of Health (NIH) exemplifies repurposing an outdated 1960s facility to house a complex and demanding research program. Planning and design for the cGMP began in 2017 with BIM occurring in 2021. Construction on the $29 million project began in 2021, and is expected to be finished in July 2023.
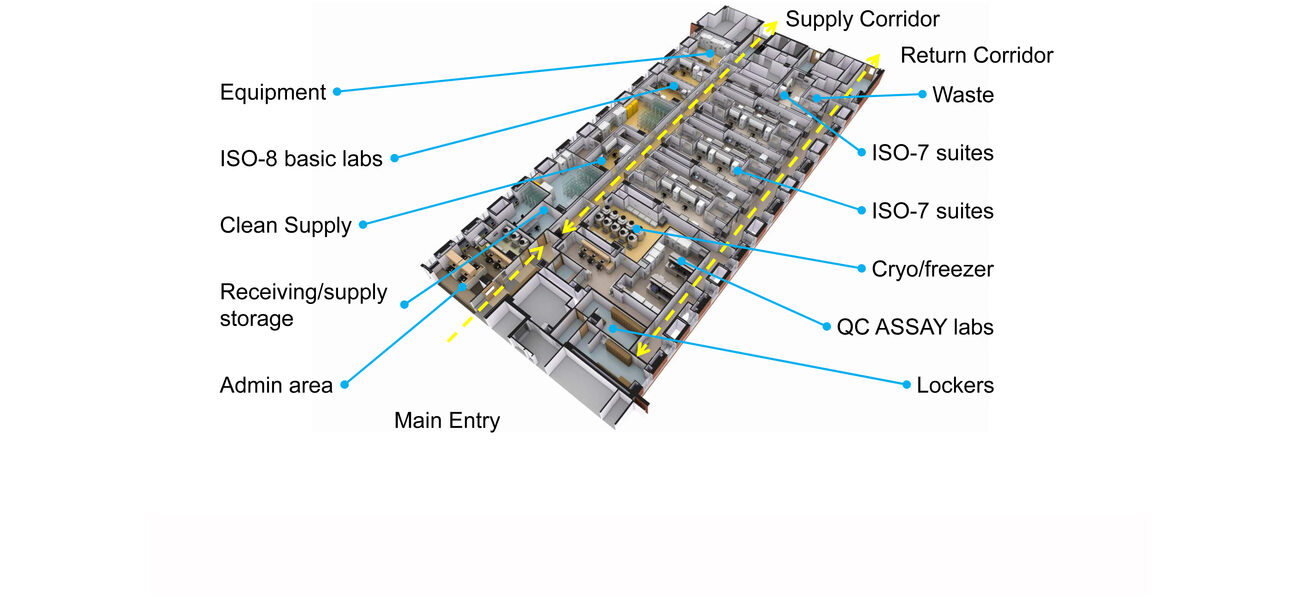
The 250,000-sf E-Wing renovation features a cGMP space on the 12th floor of the 16-story building that presented significant challenges to realize the project in a seemingly impossible location.
“We talked to the users about their research and development processes and necessary ancillary activities,” says Lucy Loukanova, science and technology market leader at Affiliated Engineers Inc. “We carefully distinguished between what should be located in the cGMP space and what should not.”
The NIH intended to locate the cGMP cell processing space on the third floor with 11 ISO 7 tissue culture rooms and one infectious disease suite. However, further review of the third floor revealed it was too small to accommodate those labs, and did not have sufficient mechanical space for the required air handling units and equipment redundancy.
Relocating the lab to the 12th floor took advantage of the existing MEP systems on the 13th floor. All additional mechanical, electrical, and communication utilities were placed on the 13th floor and connected straight down to the spaces they serve. This resolved the height limitation that prevented ductwork and piping from being installed above the ceiling as it traditionally is.
The 13th floor mechanical electrical room houses the cGMP supply air handling units, clean humidification steam system, and the supply and exhaust terminal air distribution, as well as the air handling units and respective main supply ductwork feeding various labs and offices on the 10th and 11th floors.
Multiple exhaust duct risers, serving the entire wing and starting from the two basement floors, travel vertically through the 13th floor shafts, further constraining the floor space available for MEP equipment placement and circulation. The 14th floor houses the exhaust heat recovery units serving the base building.
“Placing the MEP systems on the 13th floor means all of the maintenance work can be done on that floor without impacting the research on the 12th floor,” says Loukanova. “The 12th floor cleanroom light fixtures are removable, which gives easy access above the ceiling for minor tweaks when necessary.”
After the specialized lab was moved to the 12th floor, the number of ISO 7 labs was reduced from the 11 requested by the NIH to 6 with an additional ISO 7 infectious disease suite, two ISO 8 prep labs, one ISO 8 equipment staging lab, and plenty of cGMP and non-cGMP support spaces, such as a training room, administrative office, records storage, and a principal investigator’s office.
Gongal says having 11 tissue culture rooms would have resulted in tiny labs with bidirectional flow of people and materials going in and out through the same doors, potentially leading to cross contamination.
Design Innovations
A cGMP space must be properly sealed to prevent air infiltration. This is especially important when creating the space inside an older concrete structure that has a poor exterior performance envelope.
A passive ventilation system between cleanroom walls and building envelope—the process of supplying air to and removing it from spaces without using mechanical systems—cools the perimeter double-wall cavity and allows natural daylight to flow into the labs and corridors through the windows. Glazing was placed in the ISO 7 labs for visual connectivity and safety.
Liquid nitrogen freezers are located on the 12th floor so that they are readily available for use by the researchers on that floor, and additional liquid nitrogen tanks are stored on the 13th floor. Liquid nitrogen changes quickly to gas form when it is released from the pressurized tanks. Due to space limitations and the potential of exposing the tanks to external environments, situating them one floor above was a perfect alternative to rolling them in and out of the labs.
“Even with a vacuum-jacketed piping, the quantity and pressure of the liquid nitrogen would be altered, if we ran the piping a long distance,” says Loukanova. “To prevent this loss, we placed the liquid nitrogen tanks as close as possible to where they are needed.”
Another innovative design feature is the intricate plumbing system, featuring double-containment piping, a leak-detection system, and wall-enclosed access panels. Area floor drains located on the 13th floor are carefully positioned so they are not directly above any of the ISO 7 or ISO 8 spaces.
The use of prefabricated cleanroom panels is another good idea, says Gongal. The panels forming the wall are 2 inches thick, and those on the ceiling are 2 to 3 inches thick.
“You can run conduits through them and run gas lines around them, so it saved us a lot space,” he explains. “The ceiling panels can hold the light fixtures and HEPA filters, so you don’t need separate hangers.”
Ceiling-mounted HEPA filters provide final-stage air filtration at the cleanroom boundary, protecting the space against any accidental particulates in the ductwork.
Special Requirements and Challenges
Cell therapy was a relatively innovative process when planning began for the NIH E-Wing.
“It needs to be implemented in a cleanroom environment, and that means there is a tremendous number of air changes based on the classification of the space to keep those particulates within the allowable FDA ranges,” says Loukanova. “It also means lower space temperature and relative humidity for both the process itself and for user comfort within the space.”
Pressure cascading from the cleanest to less clean spaces also has to be maintained, measured, confirmed, and validated at all times. With a tight building envelope, it is critical to prevent air infiltration, exfiltration, and condensation that might occur due to temperature differences. There are stringent air change requirements for ISO 7 and ISO 8 spaces.
“We had a lot of challenges in this building, because the envelope was old and the building was never meant to be a scientific space,” says Loukanova. “It was a concrete structure with 3-foot-deep beams at every column bay, so that left almost no room for utility crossing under the structural beams and above the 9-foot ceilings that we had to incorporate in the project. We also have a shaft in every structural bay, and the shaft square footage took away from the available program square footage.”
The base building ductwork is distributed such that main duct crossings are avoided on the floors. The south and north corridors are used for the supply ductwork, while the exhaust risers are located on the perimeter and alternating with supply risers in the central bays.
The NIH presented the engineers and other team members with specific requirements, including but not limited to:
- Ultimate flexibility for different uses of the cleanroom in the future;
- Reliable, redundant MEP systems;
- A sustainable facility to last 30 to 40 years;
- Room sight access to air-balancing devices;
- All service lines for power, building automation, emergency, and security systems must be encased in hard conduit.
“The biggest challenge was obviously to make the renovation fit into the building,” says Gongal.
By Tracy Carbasho
| Organization | Project Role |
|---|---|
|
National Institutes of Health
|
Owner
|
|
Perkins&Will
|
Architect
|
|
Affiliated Engineers, Inc. (AEI)
|
ME Engineer
|
|
Whiting-Turner
|
Contractor
|
|
GES/CFR Engineering
|
Plumbing Engineers
|
|
AVETS
|
Structural Engineering
|
|
Convergent Technologies
|
Audiovisual
|
|
Thornton Tomasetti
|
Protective
|
|
WDP & Associates
|
Envelope
|
|
Genesis Planning
|
Process
|
|
Wayfinding Associates
|
Wayfinding
|
|
Lerch Bates
|
Vertical Transportation
|
|
NIKA/Veterans Engineering
|
Life Safety
|