High-performance core facilities, spurred by proliferating cross-disciplinary investigations and technological advances, can benefit from the long-standing focus on flexibility that has generated so many design efficiencies in the traditional research lab, says Randy Kray, senior vice president and science and technology director of programming and planning at HOK.
From the vivarium to the imaging suite to the nanofabrication cleanroom, core facilities—defined by the NIH as “a centralized shared resource that provides access to instruments, technologies, and services, as well as expert consultation to investigators supported by the core”—have typically been purpose-built around a specific function or tool. Three major trends are causing that to change, says Kray: accelerating collaboration among scientific disciplines; inevitable, yet often unpredictable, advances in technology; and the growing need for new management and financial models.
Complex and costly, today’s core facilities surpass the financial reach and expertise of individual PIs or research programs. Multiple funding sources are necessary to support the core facility, and the right design decisions are essential to maximize the institution’s investment return.
“Institutions are struggling with where to put the types of cores they want,” explains Kray. “They are trying to predict potential uses early on, so these don’t become static spaces where change can be very expensive.”
One solution is to design the previous one-off spaces around an adaptable core platform based on a universal core-and-shell strategy independent of the research tools. When built on a foundation that incorporates key common denominators, the core facility is primed for changes in use without excessive cost. An adaptable core platform allows the space to shift and grow to suit the needs of the institution over time.
“You can’t build in complete flexibility,” says Kray. “A high-performance facility can’t shift from magnets today to genomics sequencing tomorrow. Still, it’s important to have a methodology for making intelligent decisions in key areas, like vibration criteria and air volumes, for future flexibility.”
Core Types and Service Models
Working on projects for several research clients, Kray and colleagues Chirag Mistry, senior lab planner and project manager with HOK’s science and technology group, and Kevin Brettmann, director of science and technology for JE Dunn Construction, have observed and responded to patterns and trends in core facility design.
“Core facility design is now being driven by a convergence among biology, chemistry, the physical sciences, and engineering,” says Kray. “This interdisciplinary approach to problem-solving is critical to the success of both the institution and the research.”
Kray, Mistry, and Brettmann have identified six general types of core facilities, which has enabled them to develop guidelines for infrastructure requirements, flexibility and adaptability, and cost and management techniques:
- Vivarium, which must provide containment or be pathogen-free, housing small and large animals for basic research, discovery, and efficacy/GLP functions;
- Diagnostics, equipment categories ranging from MRI/PET, proton/cyclotron, and CT/X-ray to ultrasonic and spectrometry, much of which requires magnetic, EM, and RF shielding;
- Imaging equipment—scanning electron microscope, transmission electron microscope, scanning tunneling electron microscope, MRI/NMR, X-ray crystallography, optical/laser, or atomic force microscope/probe—all requires specialized environments;
- Cleanroom fabrication, whose functions include research, development, or formulation for biochem/small molecule, biologics/large molecule, or cGMP/pilot scale operations;
- Nanofabrication, based on cleanroom technology, such as tissue engineering or bioengineering, in applications ranging from drug delivery to device development;
- Informatics (bioinformatics, genomics), with both cloud storage and local storage, well-suited for integration within research floors as a place of collaboration.
These six types can also be grouped by service model, defined as either equipment-driven, resource-driven, or data-driven, according to Mistry.
Equipment-driven cores include cellular imaging, nuclear imaging, and diagnostics, typically with special infrastructure needs such as a low-vibration, low-EMI environment for advanced imaging, or the hazardous processes and safety issues of nanofabrication. Still, from a cost-recovery standpoint, these high-end tools are “perhaps the easiest to manage,” with a simple accounting model, driven by equipment output and cost. “It is easy to charge a user for an MRI scan,” notes Kray.
Resource-driven cores include in-vivo/transgenic and GMP/fabrication facilities. Changes in this area are exemplified by the shift from the traditional mouse model to the larger animal model in the vivarium; and in the emerging field of embryonic cryogenics, which is driving the need for more freezer capacity, putting an added load on the equipment core.
Data-driven cores—bioinformatics and genomics—require dry research space, which is much less infrastructure intensive. They can thus be more easily integrated into the research facility, a characteristic that spurs desirable collaboration among PIs.
Infrastructure Requirements
Kray and his colleagues have created detailed tables that correlate various core functions with six different categories of infrastructure requirements: building structure, building services, isolation shielding, daylight and views, building flow, and safety and security.
Patterns or common denominators stand out when the functions are mapped against requirements for things like power demand, cooling capacity, or security. For example, from a structural perspective, the vivarium, imaging, and GMP and nanotech cleanrooms are compatible in terms of floor-to-floor heights. The vivarium and cleanrooms share the need for increased ventilation. This data can be an invaluable decision-making tool as institutions balance flexibility with cost.
“You want to find the sweet spot of the science and then develop a common platform that could be adapted for multiple uses, say by adjusting air flows, without too much of a financial premium,” says Brettmann.
“The goal is to identify common criteria where there might be the opportunity to design an adaptable core platform,” echoes Kray.
Financial Dimensions
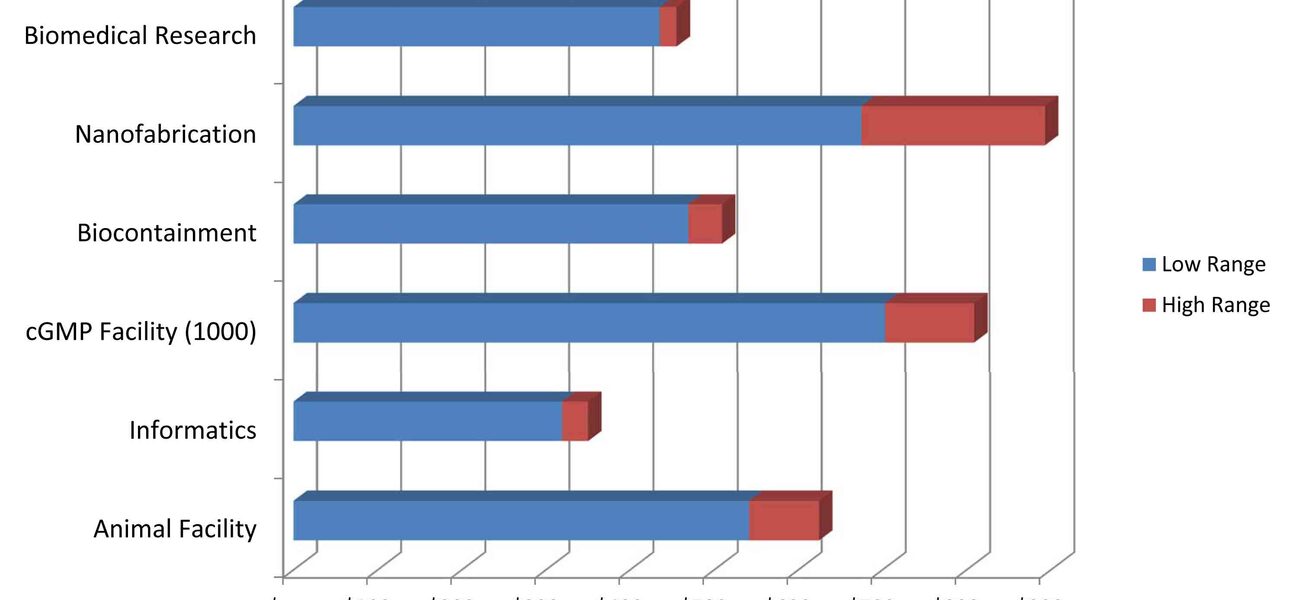
Kray and his colleagues cite data that benchmarks construction costs for six different facility types: biomedical research, nanofabrication, biocontainment, cGMP 1000, informatics, and animal. The range progresses from roughly $300 per sf for informatics to almost $900 per sf for nanotech space.
Despite the many variables, “one thing we can be certain of is that this is expensive real estate,” says Kray, adding that funding tends to be uncertain, based on a “cup-in-hand” approach.
“Institutions have to go to their leadership and their boards and ask for money to invest in capital-equipment-intensive cores. As an internal capital investment, the profit from external sources is limited, and there isn’t a great cost recovery model, as cores only charge actual costs back to their internal end users.”
Cost management is further complicated by the fact that the pace of technological advance is much more rapid than equipment depreciation.
“It might take 10 years to depreciate a microscope, but it is quite likely scientists will want a microscope that is much more advanced and sophisticated at their disposal,” says Kray.
In fact, the core facility and its equipment are fundamental to the success of the institution in attracting faculty.
“Researchers want to use the highest and the best technologies,” notes Kray. “That is why they are coming to your institution, bringing funding dollars with them. Core spaces are critical to competing for grants.
“Many of our clients look at their competitors’ core facilities and grant funding before deciding whether to invest in trying to receive grant money in a particular area of research. In general, if you can create one core rather than multiples, these cores are then not competing for the same research, which levels the playing field. The ROI comes in the success of recruiting and competing for grants.”
Consolidation and Co-Location
As institutions refine their focus on specialty areas and core competencies, and thus integrate their research efforts, core facility consolidation becomes an attractive option.
Kray points out that co-located equipment offers the financial advantages of higher utilization and less duplication. It can form the basis of partnerships that extend geographically beyond the campus, such as a genomics facility that services local or state agencies. These arrangements contribute to more realistic cost recovery and encourage synergies among various entities.
Some core or equipment types lend themselves to co-location while others do not. One example of a core facility fully integrated within the research neighborhood can be found in the new Francis Crick Institute in London. This 700,000-sf biomedical research facility not only co-locates histopathology, cell services, high-throughput screening, and flow cytometry, but also features collaboration space around the core that promotes multi-disciplinary projects.
Other core spaces should be distributed because of the science needs, notes Kray. For instance, the short life cycle of cells makes cellular imaging best done in close proximity to the research.
Overall, the co-location of high-performance equipment within a core has many benefits. The cluster of expertise furthers new ways to apply these tools to the science, for example using a PET CT scanner in the effort to modify a gene trait in a mouse.
The institution must carefully consider the trade-offs to find a balanced value proposition.
“The question of what bandwidth of performance should be built into the core space and where it should be located includes many variables,” says Kray. “Microscopes need very low vibration, and they should be near researchers. They have to be in the basement, but that will not be possible if the basement hasn’t been built to the necessary standards. If the microscopes can be co-located, one person could manage three or four of them, an opportunity for cost savings.
“There is definitely a market need for this type of thinking,” he says
Dual Management
One successful approach for some institutions has been the creation of a dual management arrangement for their cores, with positions for both a technical director and a scientific director. The technical director is 100 percent dedicated to the core, in charge of the product line, and responsible for throughput, output, quality, and the regulatory issues.
The technical director reports to a scientific director, typically the staff researcher who best understands the core technology. This post is only partially funded by the core—perhaps at a level of 50 percent. The rest of the individual’s time is spent on research.
“The scientific director is the external face of the core to other researchers and other external partners,” says Kray. “This has been found to be a highly successful model. It effectively addresses the integration of science and core expertise.”
The Adaptable Core Platform
With change the most predictable characteristic of today’s scientific research, the adaptable core platform resolves many planning issues, including helping an institution maximize its investment return. Strategically planning core space independent of the current tool or technology is a robust approach to preparing for the adaptive reuse demanded by the growing convergence of disciplines in scientific investigation.
“We need to think as much about making an investment in this high-performance space as we do about generic lab space. The industry hasn’t focused on flexibility in these areas as much as on the space allocated to principal investigators, and it is time for a new value proposition,” concludes Kray.
By Nicole Zaro Stahl
This report is based on a presentation Kray, Mistry, and Brettmann made at Tradeline’s Academic Medical and Health Science Centers 2013 conference
| Organization |
|---|
|
JE Dunn Construction
|