The University of Massachusetts Amherst (UMass Amherst) recently completed the construction and fit-out of their new Life Science Laboratories after receiving a $95 million grant from the Massachusetts Life Sciences Center (MLSC)—a quasi-state agency dedicated to growing the state’s life sciences industry. The new interdisciplinary research wing features state-of-the-art equipment and core resources that will be shared across multiple research teams and industry partnerships. While the new core labs were built into a pre-existing shell with an open floorplate and operational MEP, the final design was driven by the cost-intensive equipment list. Since the agency grant designated a specific amount of funding for the equipment, the type of equipment was known but exact model and vendor was not known before many of the other design and programming decisions were made.
Knowing what equipment was going to be installed helped guide the design and buildout of the core labs, but it also created unanticipated challenges relating to installation, operations, support agreements, and costs. Therefore the process had to address these challenges with flexibility in mind during design, and cost allowances during construction.
“One of the biggest drivers in developing these core labs was the equipment list,” says Peter Gray-Mullen, capital project manager at UMass Amherst. “That original grant list lived with us through design, construction, and installation of all that equipment. It was handed to every PI, faculty member, and business group as they became part of the institute. It was very helpful for us to be able to say, ‘This is what we are getting and this is what you are going to operate.’”
The MLSC grant was split into three parts—equipment dollars, capital construction costs, and a mix of startup and faculty incentives—with the intention of using the grant money to develop shared lab resources and create an industry-leading research hub in the region.
“It was a perfect opportunity for us because we already had a shelled building. And the grant helped us target and purchase the correct equipment for the fit-out,” says Gray-Mullen.
Phased Fit-Out + Equipment-Driven Planning
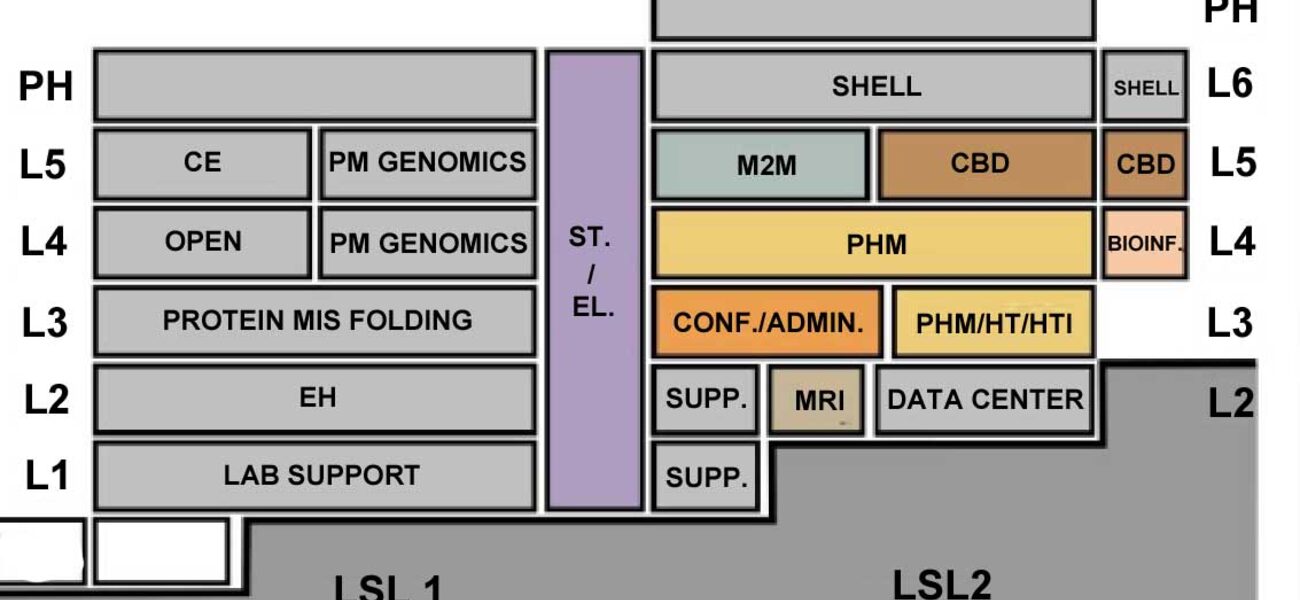
UMass Amherst originally opened the 310,000-sf Life Science Laboratories Building in 2013, but only 60 percent of the facility was built out. The remainder of the building was a shelled open floorplan with the MEP infrastructure in place. This allowed it to be fit out in a second phase with additional core labs and other cost-intensive resources as funding became available. This fit-out was the Institute for Applied Life Sciences.
Soon after the opening ribbon-cutting for the original building in 2013, UMass Amherst had another ribbon-cutting event for the $95 million MLSC grant to start the second phase of buildout.
“There was a $52 million line item in the original grant that was allocated for about 500 pieces of equipment—200 of which you would consider major pieces of equipment,” says Gray-Mullen.
One of the project’s main goals was to use the space to consolidate multiple elements of the new Institute for Applied Life Sciences that were previously scattered in different locations across campus. Ultimately, the second-phase fit-out added 22 more core labs to the Institute for a total of 30 labs featuring a wide range of shared resources.
Equipment-Driven Design
The equipment list was a spreadsheet that detailed every piece of equipment along with its technical requirements and location within the building. Equipment not purchased yet was given dimensional and utility services assumptions, including size, clearances, weight, power, and gas connections.
“One of the most important parts of the equipment spreadsheet was that each piece of equipment was given a number, because we needed a ledger number for the grant. That number ended up on our floorplans, and everybody started talking about that piece of equipment based on its number. Of course, it was assigned someplace in the building and eventually had a purchase order attached to it. Those equipment reference numbers are still being used for operations and maintenance, so that one number has gone a very long way for us,” says Gray-Mullen.
The newly opened wing features a wide array of advanced research equipment and lab types including MRI, confocal microscopy facility, additive fabrication, nanofabrication, and other vibration-intensive pieces of equipment that required careful placement.
“We determined where some of this equipment needed to sit structurally and then made sure that it didn’t move too far from where it was originally proposed. That helped us dictate a little bit of what we were doing with the core labs,” says Gray-Mullen.
Another notable structural feature of the facility is a room-sized calorimeter—one of only 26 such facilities in the world. It contains two sealed metabolic chambers of different sizes that can precisely measure changes in human metabolism based on diet, exercise, and other factors.
Challenges and Complications
Receiving a check to purchase the cost-intensive research and imaging equipment in advance was a huge benefit, but it also created a number of unforeseen challenges and complications. Some of these challenges arose from the fact that, as a newly established institute, the leadership and structure of the organization were still being created as the facility was being built.
“This was a new institute with a new way of operating across the campus. And it was really the first large-scale shared core lab we had on campus. The faculty members who served as surrogates have done research with these types of specialized equipment. They know what they want to get out of it, but what does that mean for the rest of the core lab users? We didn’t have a structure in place to give us easy answers for some of the questions that the design team was asking,” says Gray-Mullen.
This inevitably led to change orders, installation delays, and unexpected costs. Complicating things was the fact that much of the equipment was also customized, so it required more than just a plug-and-play approach to installation.
“Probably the hardest part about this was that construction was done three to six months before a lot of the large MEP-intensive equipment arrived. So, we didn’t have a lot of technicians or core directors who could assist with hooking that equipment up,” says Gray-Mullen.
These complications extended to things like the metabolic chambers, which required specialized MEP connections and structural features; and installation of the MRI, which resulted in more than $500,000 in change costs.
“We had one piece of equipment that went from what we considered to be a normal fume hood level of exhaust of 300 CFM, up to 3,000 CFM with temperatures up to 450 degrees Celsius. So, we had a few surprises,” says Gray-Mullen.
Since the institute was so young, there was no staff available to operate many of the large pieces of equipment when they arrived, and installation was often left to contractors. This also meant warranty coverage on some of the equipment began prematurely—as soon as it arrived and was uncrated, but months before it became fully operational.
“It took us awhile to find the right person to lead things. We had some surrogate staff along the way, but that’s not the same thing as having the actual staff that is going to end up operating the facility. We did our best to utilize core lab directors doing similar work elsewhere, and engage their knowledge to get things going until we found the right person,” says Gray-Mullen.
By Johnathon Allen