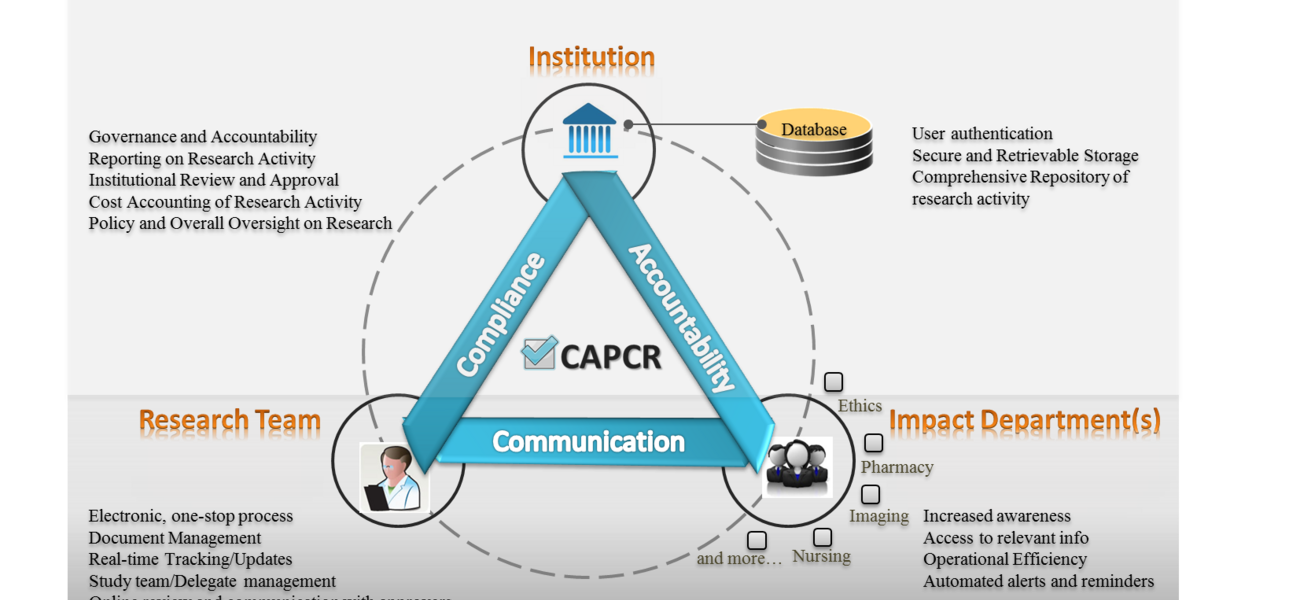
The Coordinated Approval Process for Clinical Research (CAPCR) is a web-based application that streamlines the approval process required to conduct research using human subjects. Designed to help researchers and hospital staff navigate the process of coordinating and tracking clinical research within a hospital system, CAPCR gives an institution time to plan for the infrastructure a line of research will necessitate, and to mitigate any associated safety hazards. CAPCR automates the approval process, avoids duplication, and creates an online repository for study-related information, allowing faster approval times and greater access to information. It also eliminates the need for paper application forms, helps researchers ensure they have the necessary approvals for their studies, and provides an efficient way to obtain authorizations from multiple departments and track approvals online. The system is being marketed as a tool for use in other hospitals.
CAPCR was created by an internal team at Toronto’s University Health Network (UHN), the largest research hospital in Canada, which consists of five research institutes at four different hospitals: Toronto General Hospital, the Toronto Western Hospital, the Princess Margaret Cancer Centre, and the Toronto Rehab Institute. Research Support Services (RSS), a unit within UHN, provides skills training, program development, and IT solutions to support UHN’s 800 researchers and manage about 818,000 sf of research space. MedRIST (Medical Research Integrated Solutions and Tools), a revenue generation unit within RSS, manages RSS’s business portfolio by expanding its services beyond UHN. CAPCR, the newest item in the MedRIST portfolio, was launched in February 2012 after 18 months in development.
“Departments in the hospital providing support to human research subjects were finding they didn’t have great systems to manage this relationship. Everything was based on signatures and paper documents, and very little of the process was online. We needed one system to close the loop,” says Paul MacPherson, director of grants, contracts, and ethics review services at UHN.
All research involving human subjects, performed under the auspices of a research institution, requires institutional authorization from all the departments involved or impacted by the study. At most hospitals, the main approval route for such research is review by the Research Ethics Board (REB), an independent board that reviews all proposed studies to make sure they will not harm human subjects. Each study is also subject to review by various other departments, such as contracts, finance, and facilities management. In a large research institution, there is often no easy way to say “yes” or “no” to a research study, and the process can take a long time.
“We needed a standard way to get everyone involved to say we are providing or rescinding institutional authorization,” says MacPherson, whose department supports the REB. “We frequently heard that researchers wanting to do clinical research here were frustrated because it was difficult to get the appropriate approvals in a timely manner. Also, as an institution with 16,000 employees at four hospitals and lots of clinical research going on, we needed a better way to coordinate the process.”
CAPCR not only puts in one place all the necessary documentation to manage research studies involving human subjects, it also creates an online repository for document storage and research approval information, allowing regular updates and compliance checks. The benefits of CAPCR include:
- Enhanced communication
- Shorter approval timelines
- Single, “smart” application form
- Electronic submissions and signatures
- Reporting and tracking features
- Electronic, 24/7 access
- Helps the institution assess the overall impact of a particular line of research
To build CAPCR, Ajay Pillai, manager of MedRIST, and the CAPCR project team gathered documents from the approximately 25 hospital departments that are potentially involved in approving clinical research studies—including ethics, finance, grants and contracts, nursing, pharmacy, lab services, and facilities management—and extracted about 900 possible questions related to clinical research approvals. Based on meetings with each department, they spent about three months narrowing this list to create 70 “trigger” questions, grouped by department. These yes-or-no questions identify the sections a researcher must complete in order to have a study reviewed by all the necessary people, creating customized online forms for research proposals.
“Ajay and team literally spent days locked in committee rooms with a table full of forms, figuring out the best way to group these questions,” says MacPherson. The team also worked with developers to create a prototype of CAPCR, prior to launch, to demonstrate how it would work and make sure they had buy-in from each department. “We had to validate the new forms and questions with each department to show them we had gotten the questions right, and grouped them properly to provide the information they needed to conduct their reviews,” says Pillai.
Compliance, Accountability, and Communication
A researcher submitting an application for clinical research logs into CAPCR and answers specific trigger questions, such as: Is there imaging? Is there blood collection? Is additional space required? Will you hire additional staff? Each “yes” response prompts a different application section to be required. Departments involved in institutional oversight for each study receive notification and relevant forms, providing increased awareness and early access to information, and prompting automated alerts and reminders to help keep the process on track. “The system directs them to the right department, so anyone who needs to review the study and provide approval will see it. Before CAPCR, the researcher was responsible for submitting forms to each department. Now it is all within the system,” says Ian McDermott, senior director of MedRIST. For the research team, CAPCR provides online records and document management, online tracking and real-time updates throughout the review process, and easy communication with reviewers. Some departments or individuals want to be in the information loop, but don’t need review oversight or approval. A pharmacy reviewer, for instance, can log into CAPCR and see only submissions related to that department, while the finance department may view information about cost analysis and impact. Some departments receive notification only when a new study is approved. “They see the studies they need to review, get questions asked and answered, and see the information and documentation necessary to make the review decision. It’s a pretty dynamic process,” says Pillai.
McDermott, whose department is responsible for safety, says CAPCR makes it easier to identify potential problems or hazards early in the approval process so there are fewer surprises. For instance, studies using research samples require heightened precautions due to the potential for human pathogens in the samples, compared to using clinical samples, from an identified source. “A lot of the clinicians did not realize the difference in safety requirements, but CAPCR makes it clear. It takes a researcher through the process so all approvals from each department involved are in place prior to granting institutional authorization.”
Through MedRIST, UHN is currently negotiating contracts with three other Canadian hospitals interested in using CAPCR. The base rate is about $25,000, which includes hosting and maintenance fees. Pillai says most hospitals can expect it will take about six months to get the system up and running. CAPCR is created using modules that can be customized for other institutions. It can also be integrated with existing systems.
Phase one, currently in use at UHN, focuses on creating, submitting, and getting approval for a clinical study. Phases two and three, scheduled to launch in September, will focus on managing ongoing approval, including amendments and related events, and dealing with renewal or closure.
While Pillai says MedRIST has not quantified the improvements CAPCR has brought to UHN, anecdotal comments from researchers indicate the system is making the route to clinical research approvals easier. “Researchers are telling us it is a lot faster to win approval now. Before, the time between the first submission and last submission could be 75 days, but with CAPCR, you have a one-time submission. We have not yet gathered the analysis, but we have heard from our researchers that this is a much better experience,” he says.
By Mary Beth Rohde