Researchers at the Health Protection Agency (HPA) in the United Kingdom have developed a way to safely anesthetize and transfer animals from BSL-3 and BSL-4 containment to a mobile imaging facility for CT and MRI scans without breaking the containment barrier. The process allows them to obtain the high-resolution, three-dimensional images necessary for their research without building a costly suite onsite.
The HPA’s Microbiology Services Centre at Porton Down is required by the government to conduct research using a wide range of animal species to study many infectious diseases, including tuberculosis, AIDS, meningitis, and influenza.
Studying the progression of disease in animals from mice to primates helps researchers understand the type of vaccines, preventive measures, and medical treatments that may be effective in human health care. The research is enhanced by using high-resolution computed tomography (CT) or magnetic resonance imaging (MRI), both of which provide 3-D pictures to show how a disease impacts animals.
“Imaging of experimental subjects in life is a powerful way of measuring progression to disease, vaccine, therapeutic efficacy, or in defining the pathogenesis of a novel infectious agent without sacrificing a series of animals to obtain this data,” says Mike Dennis, a scientific leader for the HPA. “Besides the ethical aspect, it also brings the model closer to the clinical situation by reflecting what happens in humans.”
The use of experimental animal models allows these images to be taken at key points before and after infection, which is something that cannot be done in humans, he says.
While the imaging is extremely helpful to researchers, they face challenges associated with housing, transporting, and testing infected animals in BSL-3 and BSL-4 containment facilities, particularly when the animals are infected with airborne pathogens.
Effectiveness of Imaging
CT scans use X-rays to create high-resolution 3-D images of tissues, whereas MRIs use strong magnetic fields and non-ionizing radiation to generate high-contrast 3-D images.
“The advantage of CT over MRI is a much shorter scan time, which is good for the animals because it eliminates the need for breath-holding strategies to get good lung images,” says Dennis. “It provides less detailed images than the MRI, but is very good for imaging bone because it is an X-ray-based system. MRI has given us superb images of the lungs, but in vivo (with living subjects), its main strength is in scanning brain and soft tissues, such as the spleen and liver.”
The HPA used rhesus macaque monkeys infected with tuberculosis in a pilot study at collaborating facilities in Oxford and the United States to determine the benefits of both CT and MRI. CT images of the deceased monkeys’ lungs revealed an increased level of disease in animals that were given a higher dose of aerosol exposure. In addition, the presence of pulmonary disease was clearly evident three weeks after the animals were exposed to the aerosol, long before any clinical signs can be observed. Researchers found a direct correlation between the pathology counts and the imaging results.
“We are looking for pathological changes in the various tissues and organs, such as discrete lesions, enlargement of organs, or changes in the structure of tissues,” says Dennis. “But these are still based on a terminal readout of disease, so we really needed to progress by going into in-vivo imaging to test animals while they are still alive.”
After the HPA validated the effectiveness of imaging, it faced the decision of investing in imaging equipment and determining which imaging modality would be most suitable for the facility and its researchers.
Making Practical Decisions
The HPA wanted an imaging system that would maximize the capability for multi-project use on a variety of subjects infected with different agents. A critical consideration was ensuring that imaging could be done without contaminating the staff, equipment, or other aspects of the facility.
“We had two choices: We could purchase scanning equipment and integrate it into our facility or we could use mobile scanners,” notes Dennis. “The problem is that our current biocontainment facilities were built in the 1950s and are of sound construction but difficult to modify. Plus, we have no suitable space for the equipment and no imaging specialists on site to operate it. Imaging is a rapidly evolving technology, and we felt that if we invested in it now, it might be out of date by the time we got it installed.”
The agency opted for the cost-effective alternative of using state-of-the-art mobile CT and MRI scanners. This choice required no investment in extra facilities or technical experts to operate the equipment.
Once the decision was made to use mobile scanners, the HPA had to address the regulatory issues of working with BSL-3 pathogens, and the welfare issues of transporting animals and keeping them anesthetized during the scanning. All equipment, such as the containers used to transfer animals, has to be compatible with the imaging modalities. For example, the equipment used to transport the animals has to fit into the mobile scanner, obviating the need for technicians to move the animal and possibly cause contamination.
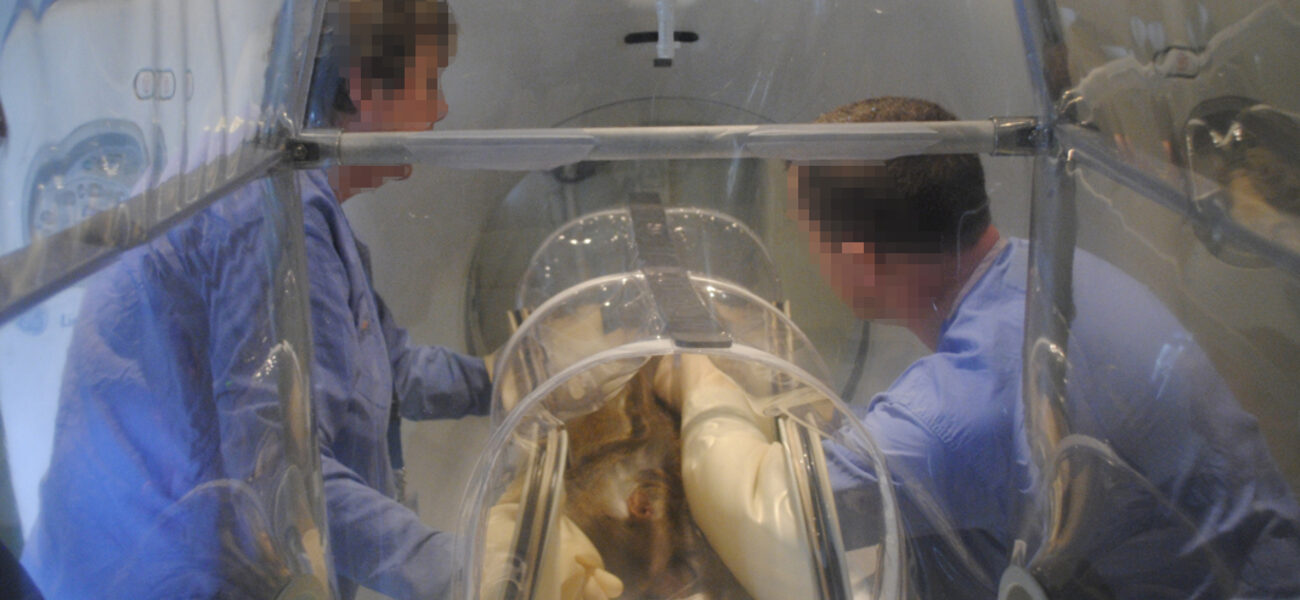
A double-skinned imaging pod, developed by the HPA, works well for transporting animals to the imaging unit, transferring them into the scanner, and then facilitating the imaging. All of this can be done while the animal remains in the contained pod, which features an outer flexible film isolator with HEPA filters and an air supply along with a second, internal containment pod. The outer skin remains uncontaminated because it connects to the outside of the holding room through a quick release docking system and the animals pass through directly into the inner pod from the room. Both pods have separate HEPA filtration and air supplies that maintain a differential negative pressure and are situated on a trolley connected to the pod. The air handling units can be briefly disconnected to allow maneuvering into the scanner. The units can then remain outside of the screened area for MRI scanning, being connected to the pods using extendable metal-free components. These precautions are not required for CT scanning, and the air handling units can be situated alongside the imaging pods. The pod containing the animal is aligned with the scanner and then moved across the scanning bed while images are taken.
“The pod concept enables greater flexibility in the use of imaging because it allows animals infected with dangerous pathogens to be moved safely from their high-containment housing facility to a dedicated scanning area without contaminating that facility,” explains Dennis. “Thus, a number of experiments could be conducted simultaneously with different infectious agents with the animals securely housed in separate high-containment facilities, but with the capability to transfer them safely for imaging at key stages in an experiment.”
The largest animals scanned at the HPA to date have been macaque monkeys, while the smallest have been mice.
Learning from Research
Having established the efficacy of scanning live, infected non-human primates, the HPA plans to apply the imaging to as many studies as possible and evaluate the future use of more advanced CT scanning modalities, such as a nuclear medicine imaging technique called positron emission tomography (PET).
The containment pod could be used for in-house equipment, as well as mobile imaging units, which is a consideration when new buildings are being constructed. The HPA has shown that the mobile units allow researchers to conduct the necessary imaging without expensive infrastructure and permanent on-site staff.
“The responsibility and cost for keeping up to date with ever-advancing technology remains with the companies providing the scanners and not with the research institute,” says Dennis.
By Tracy Carbasho
This report is based on a presentation given by Dennis at Tradeline’s 2012 International Conference on Biocontainment Facilities.