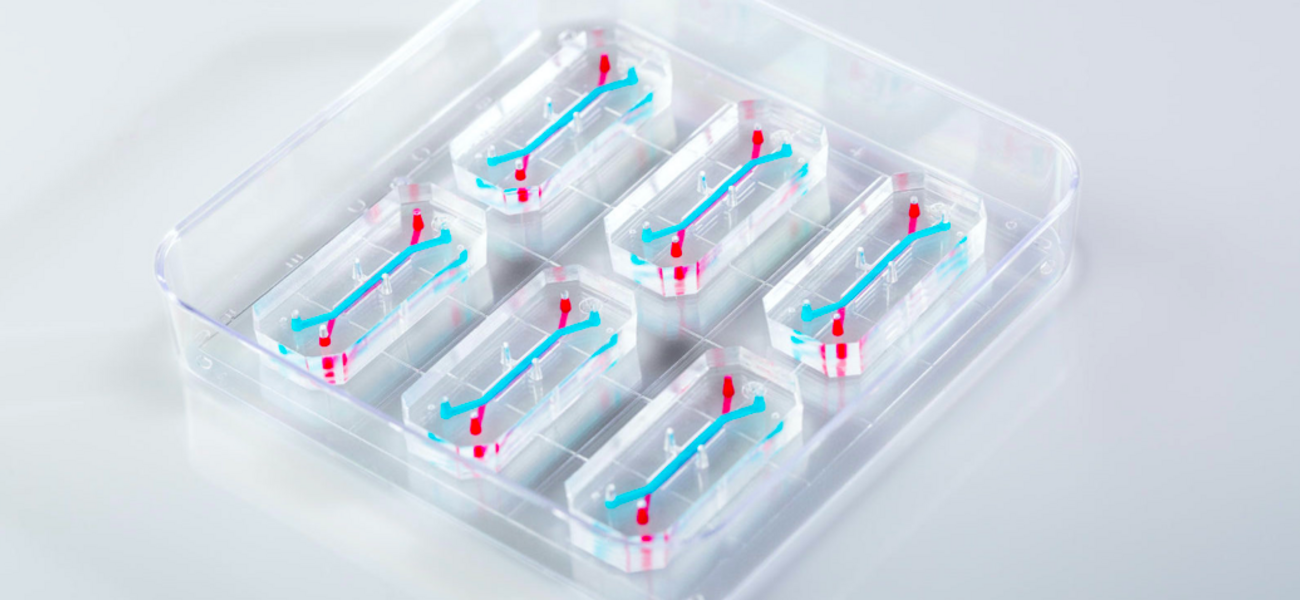
The United States Congress passed Senate Bill 5002 in late 2022 to allow methods other than animal testing to be employed to establish drug efficacy and safety. Dubbed the FDA Modernization Act 2.0, the legislation supports the inclusion of cell-based assays, computer models, and microphysiological systems such as organ chips in preclinical testing operations. The bill also rescinds a stipulation that animal studies must be used when seeking a license for a biological product that is biosimilar or interchangeable with another product. By easing regulatory requirements for animal testing, the Act will enable researchers to leverage these novel technologies to accelerate the development of human therapeutics while reducing the costs and challenges associated with animal models.
FDA Modernization Act 2.0 Allows Alternatives to Animal Testing for Drug Development
Washington
Source